Meksidol: instructions for using the medicine, structure, Contraindications
Active material: эtilmetilgidroksipiridina succinate
When ATH: N07XX
CCF: Antioxidant drugs
ICD-10 codes (testimony): F07, F07.2, F10.3, F45.3, F48.0, G40, G45.0, G93.4, I61, I63, I67.2, I69, K65.0, K85, S06, T43.4, T90
When CSF: 02.14.06
Manufacturer: Pharmasoft SPC Ltd. (Russia)
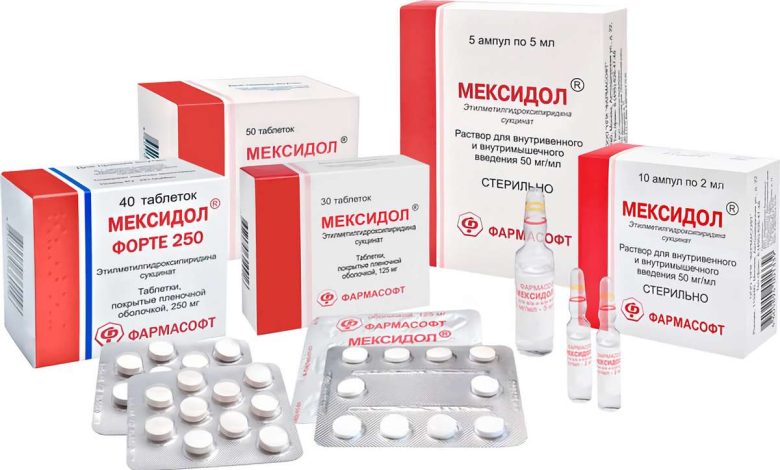
Meksidol: dosage form, composition and packaging
Solution for in / and the / m colorless or slightly yellow, clear.
| 1 ml | 1 amp. | |
| эtilmetilgidroksipiridina succinate | 50 mg | 100 mg |
Excipients: sodium metabisulfite, water d / and.
2 ml – glass ampoule (5) – contour cell package (1) – packs cardboard.
2 ml – glass ampoule (5) – contour cell package (2) – packs cardboard.
Solution for in / and the / m colorless or slightly yellow, clear.
| 1 ml | 1 amp. | |
| эtilmetilgidroksipiridina succinate | 50 mg | 250 mg |
Excipients: sodium metabisulfite, water d / and.
5 ml – glass ampoule (5) – contour cell package (1) – packs cardboard.
5 ml – glass ampoule (5) – contour cell package (2) – packs cardboard.
Meksidol: pharmachologic effect
Antioxidant drugs. It has antihypoxia, membranoprotektornym, nootropic, anticonvulsant and anxiolytic effects. The drug increases the body's resistance to the action of various damaging factors, kislorodzavisimym to pathological states (shock, hypoxia and ischemia, cerebrovascular accidents, alcohol intoxication and antipsychotics / neuroleptics /).
Meksidol® It improves brain metabolism and blood flow to the brain, improves microcirculation and blood rheology, reduces platelet aggregation. It stabilizes the membrane structure of the blood cells (erythrocytes and platelets) when hemolysis. It has hypolipidemic effect, reduces total cholesterol and low density lipoprotein.
The mechanism of action Mexidol® due to its anti-hypoxic, antioxidant and membrane action. The drug inhibits lipid peroxidation, It increases the activity of superoksidoksidazy, increases the ratio of lipid-protein, reduces the viscosity of the membrane, increases its turnover. Meksidol® modulates the activity of membrane bound enzymes (calcium-independent phosphodiesterase, adenylate cyclase, acetylcholinesterase), receptor complexes (benzodiazepine, GABA, Acetylcholine), which increases their ability to bind to ligands, It helps to preserve the structural and functional organization of biological membranes, transport of neurotransmitters and improve synaptic transmission. Meksidol® increases the concentration of dopamine in the brain. Causes increased compensatory activation of aerobic glycolysis and decreased inhibition of oxidative processes in the Krebs cycle under hypoxic conditions with increasing ATP and phosphocreatine, activation of mitochondrial functions energosinteziruyuschih, stabilization of cell membranes.
Meksidol: pharmacokinetics
Absorption
With the introduction of Mexidol® doses 400-500 mg Cmax Plasma is 3.5-4.0 ug / ml achieved for 0.45-0.5 no.
Distribution
After the / m of the drug in the blood plasma is determined for 4 no. The average retention time of the drug in the body is 0.7-1.3 no.
Deduction
Excreted in the urine mainly in glyukuronokonyugirovannoy form and in small quantities – in unchanged form.
Meksidol: testimony
- acute stroke;
- encephalopathy;
- vascular dystonia;
- mild cognitive disorders of atherosclerotic genesis;
- anxiety disorders in neurotic and neurosis-like conditions;
- relief of withdrawal symptoms in alcoholism with a predominance of neurosis-like and vegetative-vascular disorders;
- acute intoxication with antipsychotic drugs;
- acute purulent-inflammatory processes of the abdominal cavity (acute pancreatitis, peritonitis) in the complex therapy.
Meksidol: dosing regimen
Meksidol® injected i / m or / (bolus or infusion). To prepare the solution for infusion should be diluted with isotonic sodium chloride solution.
Chip Mexidol® enter slowly, during 5-7 minutes, drip - with a velocity 40-60 drops / min. The maximum daily dose – 1200 mg.
At acute stroke Meksidol® used in the treatment of the first 10-14 day / drip on 200-500 mg 2-4 times / day, then – w / m 200-250 mg 2-3 times / day for 2 weeks.
At dyscirculatory encephalopathy in the phase of decompensation Meksidol® used in / bolus or infusion at a dose of 200-500 mg 1-2 times / day for 14 days, then – w / m 100-250 mg / day for the next 2 weeks.
For ESP preventing vascular encephalopathy Meksidol® appoint / m dose 200-250 mg 2 times / day for 10-14 days.
At mild cognitive impairment in elderly patients and anxiety disorders Meksidol® appoint / m dose 100-300 mg / day for 14-30 days.
At alcohol withdrawal syndrome Meksidol® administered at a dose of 200-500 mg / drip or / m 2-3 times / day for 5-7 days.
At acute intoxication antipsychotics Meksidol® introduced in / dose 200-500 mg / day for 7-14 days.
At acute purulent inflammation of the abdominal cavity (acute necrotizing pancreatitis, peritonitis) Meksidol® administered on the first day in the preoperative, and in the postoperative period. Dose depends on the type and severity of the disease, the distribution process, variants of the clinical course. Cancel the drug should be done gradually, only after sustained positive clinical and laboratory effects.
At acute edematous (interstitial) pancreato Meksidol® appoint 200-500 mg 3 times / day / drip (in isotonic sodium chloride solution) and / m.
At necrotizing pancreatitis mild Meksidol® appoint 100-200 mg 3 times / day / drip (in isotonic sodium chloride solution) and / m.
At necrotizing pancreatitis of moderate severity - By 200 mg 3 times / day / drip (in isotonic sodium chloride solution).
At necrotizing pancreatitis severe – dose 800 mg on the first day, at double mode of administration, further – by 200-500 mg 2 times / day, with a gradual decrease in the daily dose.
At extremely severe necrotizing pancreatitis starting dose is 800 mg / day to the relief of persistent symptoms of shock pancreatogenic, the stabilization of the state - of 300-500 mg 2 times / day / drip (in isotonic sodium chloride solution) with a gradual reduction of the daily dose.
Meksidol: side effects
From the digestive system: nausea, dry mouth.
Other: allergic reactions, drowsiness.
Meksidol: Contraindications
- acute liver dysfunction;
- acute renal dysfunction;
- childhood;
- pregnancy;
- lactation (breast-feeding);
- Hypersensitivity to the drug.
Meksidol: Pregnancy and lactation
Meksidol® is contraindicated in pregnancy and lactation (breast-feeding) due to insufficient knowledge of the drug.
Meksidol: Special instructions
In some cases,, especially in predisposed patients with bronchial asthma with hypersensitivity to sulfites, the possibility of severe hypersensitivity reactions.
Meksidol: overdose
In case of overdose may develop drowsiness.
Meksidol: drug interaction
In a joint application Mexidol® enhances the action of anxiolytic benzodiazepines, antiparkinsonian (levodopa) and anticonvulsants (Carbamazepine) means.
Meksidol® reduces the toxic effects of ethanol.
Meksidol: terms of dispensing from pharmacies
The drug is released under the prescription.
Meksidol: terms and conditions of storage
List B. The drug should be stored out of reach of children, dry, dark place at a temperature no higher than 25 ° C. Shelf life – 3 year.
