The mechanism of action of medicinal substances
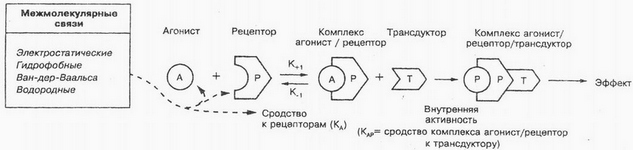
In the vast majority of cases,, to medicinal substance (Ligand) has had its effect, It must meet specific components in the body-target receptors, molecular structures, representing a protein, rarer nucleic acids, lipid or other configurations, located inside or on the surface of cells, with which it interacts, running a chain of biochemical and physico-chemical processes, causing a particular effect.
There are two kinds of membrane receptors-ion channels and receptors, G protein-related. For Example, for adetilholina and similar drugs characteristic of sodium channel. Acetylcholine interacts with a channel protein, making it conformational changes, that contribute to the Discovery Channel and the penetration of sodium ions into the cell. This process is the basis of nervous excitement. Some drugs, interacting with protein sodium channel, prevent it from opening, thus blocking the transmission of nerve excitation.
The inner part of the plasma membrane of cells aligned itself with the so-called G-protein, which provides synchronization process medicinal substance with simultaneous activation of related intracellular target proteins. As shown in Figure, drug molecule interacts with the receptor (P) on the outer surface of the membrane, which causes a conformational change of the protein receptor. Thanks to the G-protein changes its spatial structure, migrates in the plane of the membrane to the enzymes, that are in an inactive state inside the cell. Interaction of G protein with enzymes (T) makes their activation (LV/R/T). Norepinephrine, dopamine and other ligands interact specifically with receptors, G protein-related. It should be noted, that acetylcholine can interact not only with channel protein, but with the receptor, G protein-related.
For the occurrence of interaction between the ligand and bioreceptorom should be, that they have komplimentarnostju, that is, in between there must be a certain affinity, or affinitet (line sizes, spatial configuration, the presence of opposite charges and t. d.). For Example, the positive charge of exogenous ligand must conform to the negative charge of the receptor, and nepoljarnye radicals a substance can communicate with the hydrophobic sites of the receptor.
Among the physico-chemical properties of medicinal substances, affecting their interaction with the receptor, It is necessary to allocate the amount of molecules, Depending on which substance can interact with all the receptor or with an integral part. The size of the molecules of the drug depends on the kinetics of its penetration through biological membranes. Usually with an increase in the size of the molecules increases its flexibility and the possibility of the formation of van der Waals links with makromolekuljarnym partner. Besides, Stereochemistry of essential drug molecules. From, in what is form of isomeric medicinal substance, its pharmacological activity. And you need to keep in mind: the harder the conformation of the receptor molecules, the greater the difference in action stereoisomers.
The interaction of the medicinal substance-receptor is carried out at the expense of Intermolecular ties. Initially, the substance is attracted to receptor using electrostatic forces, and if there is complementarity — forms with receptor connection using physical and physico-chemical interactions (characteristic of medicinal substances, which from the body unchanged egest or maloizmenennom form) or chemical interactions (inherent in the for connections, who undergo chemical transformations in the body). The weakest van-der-vaalsovye forces take part in determining the specificity of drug interactions with biochemical rocket systems. Hydrogen bonds are involved in the processes of identification and fixing substance (Ligand) by biostrukturam. Ion arise in cases, When medicinal substances contain cationic or anionnuju group, and the opposite of the structure are in bioreceptorah. Often Ionic connection formed at the first stages of pharmacological reaction between chemicals and receptors. In such cases, the action of medicines is reversible. The importance of the education focal has Covalent bonds. With their participation, interaction flow alkylating agents with biosubstratami, as well as medicines and antidotes with metals in the formation of stable complexes-chelates, eg, unithiol with arsenic or lead-calcium tetacin. The effect of these substances is irreversible.
Besides, There is a hydrophobic interaction. Although the energy of its links is small, interaction of a large number of long aliphatic chains leads to stable systems. Hydrophobic interaction play a role in stabilizing the conformations of biopolymers and the formation of biological membranes.
Amino acid residue in a protein receptor molecule contain polar and nepoljarnye groups, which determine the formation of polar and non-polar linkages between them and the drugs. Polar group (-OH, -NH, COO-, -N3H, = O) provide education, mainly, Ionic and hydrogen bonds. Nepoljarnye group (hydrogen, methyl, cyclic radicals, etc.) form a hydrophobic context with low-molecular drugs.
Thus, drug interaction with specific receptors may be due to different chemical bonds, having different strength. So, the approximate strength kurarepodobnykh substances with holinoretseptorami for electrostatic (ion) interaction is 5 kcal/mol, ion-dipole is 2-5 kcal/mol, Dipole-Dipole is 1-3 kcal/mol, hydrogen bonding- 2-5 kcal/mol, Van-der-Waals links — 0,5 kcal/mol, hydrophobic links — 0,7 kcal per CH2-Group. Reducing the strength of the connection, depending on the distance between the atoms for the electrostatic interaction is r-2, ion-dipole-r-3, Dipole-Dipole-r-4, hydrogen bonds — r-4, Van-der-Waals links — r-7. This kind of connection can be violated, that provides the reversibility actions of medicinal substances. Stronger are Covalent bonds, that provide long lasting and often irreversible effect of substances, eg, alkylating therapy drugs. Most drugs connects to receptors reversible. Wherein, usually, the nature of the connection is complex: It can participate simultaneously ion, Dipole-Dipole, Van-der-vaalsovye, hydrophobic and other forms of communication, that is largely determined by the komplimentarnostju substances and the receptor and, respectively, their degree of rapprochement between themselves.
The strength of binding substance with receptors indicate the term «affinitet». Substances, operating on the same receptors, may have towards them varying degrees of affiniteta. When this substance with higher affinity can displace substance with lower affinity of receptor binding compounds. To determine the equilibrium between "occupied" receptors (DR), free receptors and free stuff (D) Dissociation constant is used (KD), which is determined by the following formula:
KD=[D]*[R]/[ DR]
Negative logarithm of KD (PRD) is an indicator of affiniteta. To characterize the affiniteta often use indicator pD2, t. it is. the negative logarithm of the EC50, (the concentration of the substance, It causes the effect, component 50% the maximum effect).
A variety of chemical bonds of interaction and their unequal strength, or affinitet between ligands and bioreceptorami is due to the complex structure of medicinal substances, containing different reactivity of radicals and with multidimensional volumetric form, as well as the complexity of the processes of interaction, proceeding often in several stages (phases): the formation of the complex medicinal substance-receptor; intramolecular grouping; dissociation of the complex.
Thus, pharmacological effect can cause only substances with a pronounced affinity to bioreceptoru. Pronounced effect depends on the concentration of medicinal substances and the total number of receptors.
Where substances possess sufficient internal activity, they are called agonists. Internal activity understand the ability of agonists cause a biological effect by altering the conformation of the receptor, t. it is. ability to activate the receptor ligand. It was regarded as an affinity complex-traneduktoru receptor agonist, the transformation of external signals into the Interior was called tranedukcii. Intracellular signaling underlies such processes, How to reduce muscle fibers, cell division, proliferation, differentiation, etc. It is now established, that many substances (hormones, Bioactive peptidam, nukleotidam, steroids, Molecular bioreguljatoram, etc.) cell has specific receptors. As a result of the interaction of these compounds with these specific receptors formed secondary messengers (intermediaries), that trigger a cascade of biochemical reactions.
There is a concept "partial agonists"-medicinal substances, that, by binding to the receptor, do not give maximum effect. This strange phenomenon is presumably due to incomplete (at) dependence of affinity complex medicinal substance-traneduktoru receptor. For Example, partial agonist opioid receptors nalorphine acts similar to full agonists of these receptors are morphine, Although weaker than last. At the same time when sharing their application nalorfin weakens or eliminates effects of morphine; in particular, eliminates dampening effect of morphine on the breath. Isoprenaline is a true agonist, and prenalterol is a partial agonist for β-adrenergic receptors. According to the theory of receptor, true agonist can induce maximum response, even if he interacts only with part of the receptors.
Specific receptors can have the same or different binding places for agonists and antagonists. There are different places for different binding agonists. In that case, When the agonist and antagonist have the same place of binding and receptor antagonist blocking action is completely eliminated when the concentration of agonist (the maximum effect is achieved by the agonist), the relationship between the antagonist and agonist called competitive antagonism. If the agonist and antagonist binding different, the relationship between them is defined as non-competitive antagonism. To characterize the antagonists frequently used indicator of pA2 (the negative logarithm of the molar concentration of antagonist, where to get standard agonist effect its concentration should be increased by half).
In the context of the whole organism agonists and antagonists changes or other physiological functions. Effect of antagonists by order, that they hinder the impact on specific receptors relevant natural ligands (eg, M-holinoretseptorov antagonist atropine inhibits the action of their agonist acetylcholine). Changes, which are directly related to the interaction of substances with specific receptors, denote by the term "primary pharmacological reaction, that could be the beginning of a series of reactions, leading to stimulation or oppression of certain physiological functions».
Changes in the function of organs or systems (eg, change of power and heart rate, tone smooth muscles of internal organs, secreting glands, BP and others.), caused by medicinal substance, indicate how pharmacological effects of the substance. So, for cardiac glycosides primary pharmacological reaction is oppression activity of Na + transport, K-ATPase myocardial fibers, which is regarded as a possible receptor specific for cardiac glycosides. In this connection, the broken intake K + in muscle fibers and fiber Na + exit, increases the content of Ca2 + in the cytoplasm, that promotes interaction between actin and myosin. The result of these changes is to increase the force of heartbeats, that is the main pharmacological effect of cardiac glycosides.
Prolonged exposure to agonist specific receptors are often accompanied by a decrease in sensitivity. The latter can be linked to a change in receptor, reduction in the number of (density) or violation processes, that follow receptor excitation. While pharmacological effects of agonists become less pronounced.
Thus, pharmacological effects of most drugs associated with their effects on the relevant specific receptors.
Substances with a high affinity to bioreceptoru and low intrinsic activity are called antagonists, or blokatorami, because they, without causing changes in conformation of bioreceptora, impede interaction with him endogenous and/or exogenous ligands-agonists. There are so-called "secondary or dumb receptors, which medicinal substances are associated, but not having pharmacological action. These "dumb" receptors most often present in proteins and plasma (but can also be located in tissues). Connection with "dumb" receptors leads to a decrease in the concentration of free drug, and thus to reduce the therapeutic effect.
Many modern theories, explaining the mechanism of interaction of ligand-receptor, the status of the receptors themselves, lack of proportionality between the number employed receptors and ultimate reaction, change the efficiency of signal transmission and existence stand receptors and partial agonists and t. d. formed the basis of beliefs about the mechanism of action of representatives of various groups of medicinal substances. These interactions are subdivided into interaction with the receptor and chemical interaction.
The mechanism of interaction of drugs with bioreceptorom can be represented in diagrams as follows: each ligand (medicinal substance or physiological substrate) binds to a specific receptor on certain plot. Activated receptors directly or indirectly regulate the flows of ions (1) and/or other intracellular processes (secretion or muscle contraction) or activates protein guaninnukleotidsvjazyvajushhih system (G-proteins), what, in turn, increases the activation system second mediator-enzyme. In the cytoplasm function several different second intermediaries, activating various target proteins, for example protein- kinase. Recent act on specific mediate substrates and pharmacological effect.
From the description you can see, that the effect of the drugs is carried out according to the following mechanisms:
- physiological function fabric (eg, contractile, secretory) may be subject to multiple receptors, and hence, and various ligands;
- between drug interaction with the receptor and tissue or organ may have multiple milestones, in particular the activation of receptor-related second systems resellers;
- the effectiveness of the mechanisms, responsible for the sequence of stimulus-response, as well as the density of receptors can vary from fabric to fabric.
Therapeutic effect of some drugs due to their direct (is not associated with specific receptors) chemical interaction with endogenous compounds or other mechanisms of interaction (osmolality, adsorption). So for osmotic diuretics-mannitol, urea — there is no specific receptors. These substances increase the osmotic pressure in the kidney tubules, Consequently water reabsorption is violated and increases diurez. With specific receptors does not involve action absorbent substances, forming diuretics.
Antacids (eg, aluminium or magnesium hydroxide) react with hydrochloric acid with the formation of products with weakly acidic. Chelating agents, communicating with some metals, inactive form chemical complexes.
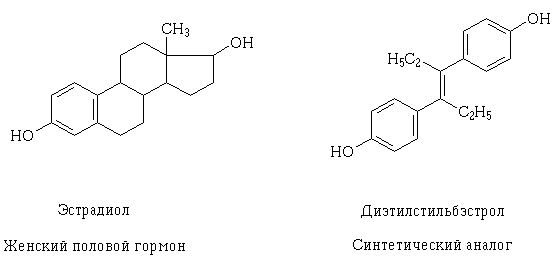
As knowledge about the structure of the receptor and the mechanism of possible farmakodinamicheskogo interaction of medicines at the cellular level, it became possible to deliberate creation, as well as an explanation, why such an effect can have drugs, different, at first sight, its structure. An example of such a phenomenon may serve estradiol and diethylstilbestrol trans - synthetic analogue of female sexual. Their structural molecules of different, but contain the same properties and dimensions of functional hydroxy groups, similarly located and oriented in space, whereby the molecules of these compounds may interact with the same receptor and exert similar pharmacological effects.
Ways to, which drugs cause those or other pharmacological effects, termed "mechanisms of action". This concept is used to explain the action of drugs on the molecular, organ and system levels. For Example, anticholinesterases mechanism of action at the molecular level is reduced to the blockade of acetylcholinesterase by reaction with an esterase and its anionic centers. At the same time, explaining the mechanism of the hypotensive effect of anticholinesterases, indicated as the reason of this effect and vasodilation bradycardia, t. it is. considering the mechanism of this effect at the organ level.
Study drug mechanisms of action are constantly, and ideas about the mechanism of action of a medicinal substance for receipt of new data can not only become more detailed, but significantly change.
