Ganirelix – drug description
When ATH:
H01CC01
Ganirelix: characteristic
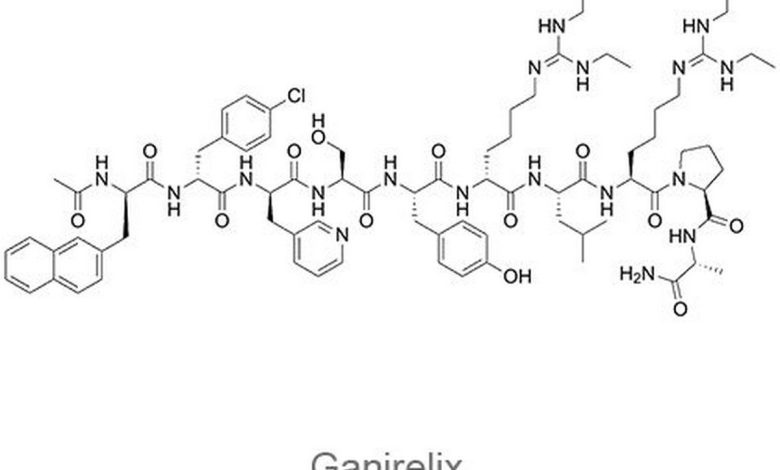
Antagonist gonadotropin-releasing gormona (GnRG). The synthetic decapeptide with high antagonistic activity to the natural GnRH. Ganirelix acetate It is a derivative of the natural GnRH with substitutions of amino acids in positions 1, 2, 3, 6, 8 and 10. Soluble in water.
Ganirelix: pharmachologic effect
Antigonadotropnoe.
Ganirelix: application
The inhibition of premature LH peak increase in women, undergoing controlled ovarian hyperstimulation in treatment of infertility using assisted reproductive technologies.
Ganirelix: Contraindications
Hypersensitivity, incl. to GnRH or any other GnRH analogue, pregnancy or suspected pregnancy.
Ganirelix: use during pregnancy and lactation
Contraindicated during pregnancy or suspected pregnancy (pre-treatment pregnancy should be excluded).
With the introduction of ganirelix acetate to pregnant rats and rabbits during the period from 7 days prior to the close to leave the period at doses up to 10 and 30 mg / day, respectively (about 0,4 and 3,2 times the human dose, on the basis of body surface area), marked increase in the percentage of resorption of litter. There was no increase fetal disorders. There were no drug-related changes in fertility, physical or behavioral characteristics in the offspring of female rats, treated with ganirelix acetate during pregnancy and lactation. The effect on bone resorption is a result of the fruits of change in hormone levels due to the properties of the drug antigonadotropnym, that can lead to loss of fruit in humans (should not be used in pregnant women).
Category actions result in FDA - X. (Animal tests or clinical trials revealed a violation of the fetus and / or there is evidence of the risk of adverse effects on the human fetus, obtained in research or practice; risk, associated with the use of drugs in pregnancy, greater than the potential benefits.)
Contraindicated during breastfeeding (unknown, PM gets there in breast milk).
Ganirelix: side effects
Adverse Reactions, have been observed in controlled clinical trials completed since the first day of application ganirelix acetate (n=794) to confirm the pregnancy using ultrasound in ≥1% of patients, to apply a PM, and they have not been directly linked to the drug administration.
Abdominal pain (gynecological) (4,8%), the death of the embryo / fetus (3,7%), headache (3,0%), ovarian hyperstimulation syndrome (2,4%), vaginal bleeding (1,8%), reactions at the injection site (1,1%), nausea (1,1%), abdominal pain (gastrointestinal) (1,0%).
In post-marketing studies it has been observed rare cases of hypersensitivity reactions, including anaphylactoid reactions after the first dose.
Congenital anomalies
According to the results of completed clinical studies of 283 Newborn, born to women, primenyavshimi ganirelix acetate, in 3 had severe congenital malformations (incl. gidrocefaliя / meningocele, omphalocele) and 18 -less severe congenital anomalies (incl. nevus, skin tags, sacral sinus, gemangioma, torticollis / asymmetry of the skull, clubfoot, extra fingers, subluxation of the hip, torticollis / high sky, umbilical hernia, inguinal hernia, Hydrocele, retained testis, gidronefroz. A causal relationship between the congenital anomalies and taking ganirelix acetate was not found.
Ganirelix: interaction
Application with other drugs has not been investigated sufficiently, so we can not exclude the possibility of drug interactions.
Ganirelix: Dosing and Administration
P /, 0,25 mg 1 once a day. Ganirelix acetate is typically administered on Day 6 of the drug FSH (controlled stimulation of ovulation FSH preparation begins on the 2nd or 3rd day of the menstrual cycle). Use of the drug should be continued daily until the date of application chGH, ie. until the formation of a sufficient number of follicles of appropriate size (preovulatory follicles) (confirmed by ultrasound), then the final maturation of the follicles can be initiated by introducing the hCG.
In the case of high ovarian response to stimulation, to prevent premature LH increase, ganirelix acetate treatment should begin with the 5th day of the application of FSH preparations. In the case of the slow growth of follicles can be postponed introduction of ganirelix (ie. starts late 6th day of application FSH preparations).
From the administration of hCG should refrain in cases, If the ovaries are the last day of FSH therapy excessively increased (may develop ovarian hyperstimulation syndrome).
Ganirelix: precautions
Can only be a specialist, with experience in the treatment of infertility.
In post-marketing studies it has been observed rare cases of hypersensitivity reactions, including anaphylactoid reactions after the first dose (cm. Side effects).
Before initiation of therapy the patient should be advised of the duration of treatment, the need for monitoring procedures and the risk of possible adverse reactions. The attending physician must be informed about drugs, that the patient has taken shortly before the start of treatment and continues to take in parallel with the appointment of the drug.
The safety and efficacy of the drug has not been established against women weighing less than 50 kg or more 90 kg.
If repeated courses of treatment should use the drug only after careful assessment of the potential risk and the effectiveness of treatment.
