Pharmacokinetic interactions and general concepts of equivalence and bioavailability of drugs
Developing common approaches use in pharmacotherapy drugs specific, must be considered, that different kinds of dosage forms have different bioavailability. If absorption medicinal substance depends essentially on its physicochemical properties, the bioavailability - largely on the properties of the formulation. In connection with this important clinical importance is the concept of equivalence, and the bioavailability of drugs.
Pharmaceutical (chemical) equivalence Medicine means, they contain the same amount of the active ingredient and comply with existing standards, whereas their inactive ingredients may vary.
The concept of bioequivalence It refers to a chemically equivalent preparations, which when administered to one patient at the same doses and on the same scheme in the blood and tissues of the active compound accumulates in identical concentrations.
The concept of therapeutic equivalence It refers to drugs, which, when administered to one patient in equal doses and the same pattern exhibit substantially the same therapeutic efficacy or toxicity; while these drugs can be nebioekvivalentnymi.
The idea of the effectiveness of the drug has always been associated with it bioavailability. Quantitative characteristic, determining the bioavailability of drugs (by definition FDA), is the rate and extent of accumulation of drug at their intended action. However, to obtain tissue samples and examine them for drug content impossible in experiment. Therefore the bioavailability of the drug substance is judged by its concentration in the blood. In practice, determine the absolute and relative bioavailability.
Absolute bioavailability It is the attitude (in %) the amount of the drug has grown deep, administered in an oral dosage form or other, to the number of vsosavshegosya
same substance, in the same dose, but as an intravenous infusion or injection, providing 100% bioavailability.
Relative bioavailability of the drug It can be measured by comparing the area under the concentration-time curve, characterizing the concentration of the substance in serum of both drugs in the identical method of administration, such as oral or rectal. Bioavailability is assessed by the concentration of drug in blood or urine, if the substance is excreted in unaltered state.
If drugs have the same bioavailability in identical conditions, they are considered bioequivalent.
According to the FDA, preparations may be bioequivalent, despite the differences in the rate and extent of absorption (when the absorption rate is not critical characteristic to achieve the effective concentration of the active substance in the body or is unimportant for the manifestation of the therapeutic effect of the drug). In some cases, the rate of absorption of the active ingredient of medicines can influence the efficacy of the treatment. One side, with slow absorption substance concentration in the blood may be below the minimum therapeutic, that does not provide the desired therapeutic effect, and on the other - at too rapid absorption, it can greatly exceed the threshold of allowable concentration, causing unwanted side effects, or toxic. Therefore, drugs, characterized by a slight difference between the minimum effective and maximum tolerated doses of the substance, are bioequivalent if, and if the degree of, and speed of the suction will be identical.
The problem is particularly acute bioavailability, when the drugs are intended for oral administration. From the standpoint of Clinical Pharmacy important difference in bioavailability of substances from different types of dosage forms. Its definition is hampered by the inability to take into account all the individual characteristics of the patient and the different properties of dosage forms. When taken orally, the drug preparations, before it reaches the systemic circulation, It undergoes a number of transitions and destination reaches fewer, which causes a low bioavailability (eg, norepinephrine, Testosterone, phenacetin et al.). The reasons for the low bioavailability of the substance may be insufficient during his stay in the digestive tract, as well as age, sex and genetically determined differences, different active patient, the presence of stressful situations, the presence of certain diseases, etc.. d. Bioavailability decreases substances and under the action of multiple factors, affecting its absorption.
Special problems arise when long-term therapy, when the patient, is adapted to one type of dosage form, transferred to another, Nonequivalent. In this case, can reduce the effectiveness of therapy, Toxic effects occur. Such cases are known when changing drugs digoxin, phenytoin, etc..
Sometimes it is possible to achieve therapeutic equivalence of drugs, Despite differences in their bioavailability. For Example, the difference between therapeutic and toxic concentrations of penicillin high, therefore fluctuations in its concentration in the blood, due to varying bioavailability of drugs, may not significantly affect their therapeutic efficacy or safety. Opposite, for drugs with a relatively small difference between the therapeutic and the toxic concentration difference in the bioavailability is important.
Since the therapeutic effect, the duration and intensity time dependence is caused by the drug concentration in blood plasma, usually consider three options - the maximum concentration of a substance in the blood, the time to achieve, and the area under the curve, resulting in the coordinates of the concentration-time.
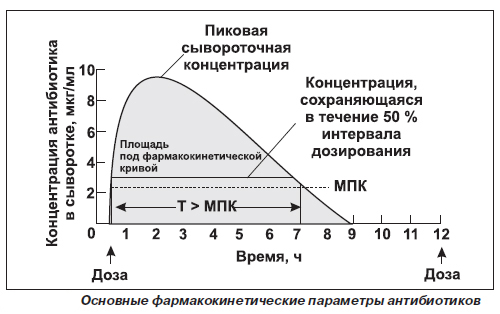
The figure shows, that the concentration of the substance in the blood increases with the speed and extent of its absorption peaks and, when the rate of material from the body becomes equal to the rate of absorption. The slower absorption of the substance, the later reached its maximum concentration.
However, an estimate bioavailability based on data of maximum drug concentration in the blood can not be sufficiently accurate, since the receipt of the substance into the systemic circulation begins its elimination. The time to reach maximum concentration depends on the rate of absorption agent and an indicator of the rate. The most important measure is the area under the bioavailability curve of concentration (PKK) from time. It is directly proportional to the total content of unchanged substance in blood plasma. To determine the PKK carried out blood sampling to total elimination of a substance. Two medicines, having identical curves rate and extent of absorption can be considered bioequivalent. If drugs have the same PAC, but differ in the shapes of the curves of concentration vs. time, they are considered equivalent in extent of absorption, but differ in its speed.
Determination of bioavailability repeated administration of the drug is preferred. PAC is measured in one of the intervals between two consecutive administrations. More accurate results are averaged in determining bioavailability within days. If the drug is excreted in the urine (mainly in unchanged form), the bioavailability can be assessed, determine the total amount for the time, equal 7-10 half-life of the substance. A more precise definition of bioavailability possible the study of blood and urine at the same time.
Thus, bioavailability and bioequivalence are the most important indicators of the quality of drugs in the characterization of their therapeutic options.
