Elements of sludge, observed in acidic and alkaline urine – Microscopic examination of urine
Kïslıy ammonium moçekïslıy often found in alkaline urine. In neutral or acidic urine it occurs in newborns and infants. Ammonium urate crystals have the form of a brownish-yellow Balls, arranged singly, pairs of clusters of different sizes.
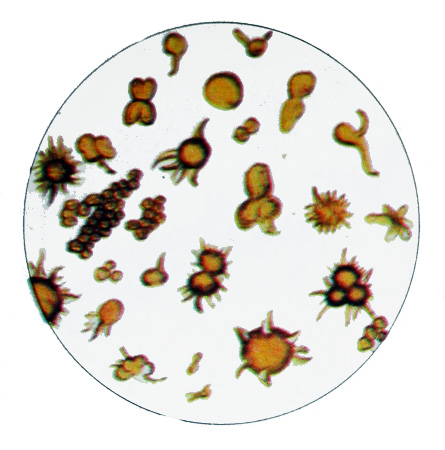
These crystals often have the styloid process, which, depending on the size and number of them give a very different shape (Datura fruit, stars).
The acidic ammonium urate dissolves during heating, and again upon cooling precipitates; by addition of hydrochloric acid and acetic acid to dissolve crystals of, forming uric acid. Mureksidnaya reaction is positive.
Oxalates can also be found in acid, and alkaline urine. These crystals often have the form of colorless, strongly refracting light octahedra (in the form of envelopes) different sizes, often very small.

Less frequently observed crystals, resembling the shape of an hourglass, as well as round and oval. Sometimes you can find larger crystals with radial growths ischerchennostyu- oxalate. In the urine of bile pigments oxalates, as well as forming elements, painted in yellow-brown color. These salts dissolve in hydrochloric acid and insoluble in alkali and acetic acid. They are often found in the urine of healthy people after eating food, rich in oxalic acid (tomatoes, shtavelya, spinach, asparagus, green beans, beet, grapes, apples, oranges and others.).
Neytralnıe phosphate They occur in a weakly acidic and weakly alkaline urine and crystallizes predominantly in the form of long prismatic wedge formations brilliant. The crystals are isolated or form rosettes, wedge-shaped pointed end it usually turned to the center. Sometimes neutral phosphates have the form of plates of irregular shape or form needle-like crystals, collected into bundles.
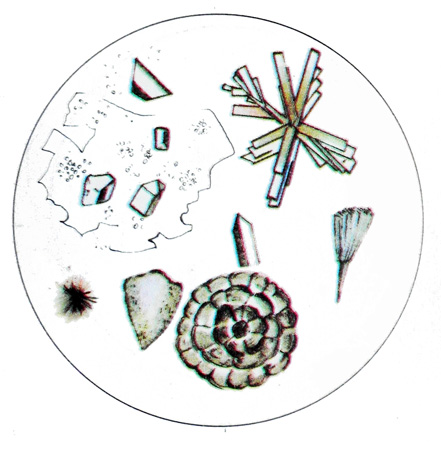
Neutral phosphates readily dissolve in acid and are insoluble in alkalis.
Calcium carbonate detected with phosphates in amorphous and crystalline form of. His colorless crystals, They have the form of concentric spheres of different sizes, located mainly in pairs and are formed in the form of gymnastic weights, crossed drumsticks, as well as small heaps. Calcium carbonate dissolves in acids to release carbon dioxide.
